Michèle Stokes PhD GAICD, Vice President – Asia Pacific at PCI Pharma Services, discusses the organisation’s world-leading status as a provider of integrated end-to-end drug development, manufacturing, and packaging solutions and its commitment to continuously evolving to meet market needs.
DELIVERING LIFE-CHANGING THERAPIES
In recent years, the rapid evolution of emerging healthcare advances and technologies has heralded the inception of a number of revolutionary medical developments.
For example, the rise of personalised medicine has seen treatments become increasingly tailored towards individual genetic profiles to improve efficacy and reduce side effects, whilst increases in mRNA technologies in the clinical trial arena, as a result of the COVID-19 pandemic, have significantly contributed to curing a breadth of rare diseases.
“Technology is advancing at a fast pace, and we are only just starting to see what artificial intelligence (AI) may bring to our industry,” introduces Michèle Stokes, PhD GAICD, Vice President – Asia Pacific at PCI Pharma Services (PCI).
A world-leading contract development and manufacturing organisation (CDMO), PCI provides clients with integrated end-to-end drug development, manufacturing, and packaging capabilities that increase speed to market and opportunities for commercial success.
“PCI brings the proven experience that comes with over five decades of delivering CDMO services, supporting more than 200 protocols across 100 countries and more than 90 successful product launches each year,” she prides.
With 30 sites across seven countries – Australia, Canada, the US, Ireland, the UK, Germany, and Spain – and over 7,000 global employees, PCI brings life-changing therapies to patients by supporting the healthcare industry’s drug product development, manufacturing, packaging, storage, and distribution needs from clinical trials through to commercialisation.
The company’s global network of innovative centres of excellence includes specialist contained facilities, which are dedicated to processing highly potent products, and renowned lyophilisation and sterile manufacturing facilities, all of which are complemented by global clinical and commercial labelling and packaging services.
“Leading technology and continued investment enable us to address global drug development needs throughout the entire product lifecycle, from manufacturing through to clinical trial supply, commercialisation, and beyond,” Stokes adds.
As such, PCI’s clients view the organisation as an extension of their business and a collaborative partner, delivering life-changing therapies together.

“Delivering life-changing therapies to patients is our number one priority, and our partners play a crucial role in supporting and achieving this”
Michèle Stokes PhD GAICD, Vice President – Asia Pacific, PCI Pharma Services
AN EXPANSIVE OPERATION
PCI supports a diverse, global client base – whether manufacturing and packaging niche personalised medicines or large-annual-volume treatments, it boasts the capability and scale to meet all manner of customer needs and deliver streamlined supply chain solutions.
“Our flexibility and experience are what differentiate us from the competition, alongside the fact that, although we are a truly global integrated CDMO, we are still small enough to care,” Stokes reflects.
Utilising its combined experience and expertise, the company guides its clients from consultancy through to delivery. One key aspect it tries to instil is that there is never a one-size-fits-all solution as every project is unique.
The vast range of PCI’s projects has contributed to its wealth of experience across all areas of product processing and associated quality and regulatory requirements.
“This ultimately provides our clients with added assurances that they are working with a company that can deliver, safe in the knowledge that our strategy is viable,” she reflects.
PCI’s specialist sites are another major differentiator, with global manufacturing capabilities, including complex formulations, highly potent solid orals, sterile liquids, and lyophilisation.

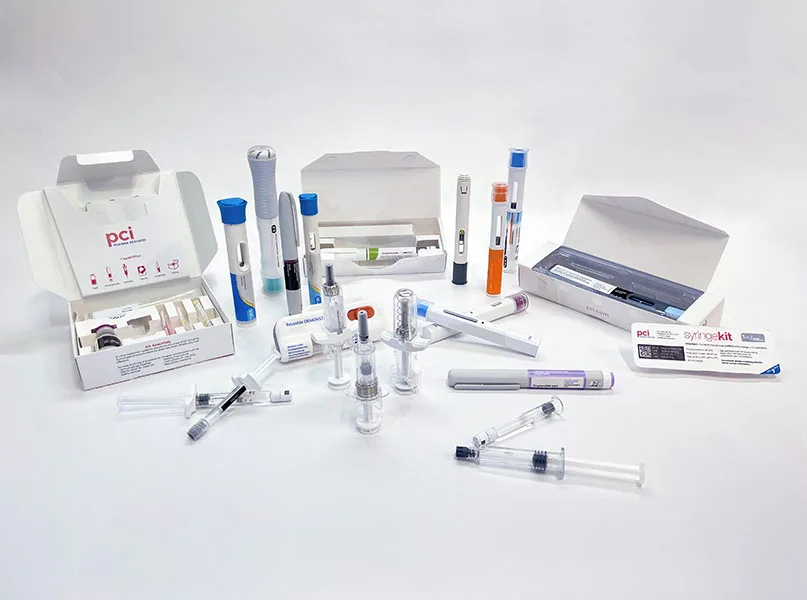
Meanwhile, its specialist global clinical and commercial packaging solutions across multiple delivery formats range from bottles and blister packs to advanced drug delivery and drug-device combination products, including vials, pre-filled syringes, and autoinjectors.
“In this way, we can find a home for just about any project at any scale, from early-stage development through to global commercialisation. Our global network ensures our clients have an in-region CDMO partner – be that in Europe, North America, Canada, or Asia Pacific,” Stokes excites.
Meanwhile, at the forefront of PCI’s digital transformation is award-winning digital supply chain management platform, pci | bridge ™, which provides clients with real-time data insights into their clinical and commercial project portfolio across the PCI network.
Production and order management data, inventory and distribution statuses, and customised reports can be viewed to make informed decisions regarding the supply chain.
As the technology continues to develop, PCI’s clients will gain expanded visibility into the product lot release lifecycle, alongside a new demand planning and forecasting submission interface and the introduction of digitised solutions, including integrations with Smartsheet and DocuSign.
“This tool has enhanced our relationship with our customers, removing manual processes and providing the data they need, when they need it,” she confirms.

WORLD-CLASS SERVICES
As a world-class CDMO, PCI relentlessly strives to deliver better health outcomes for the patients it serves by combining its experience and expertise in science, manufacturing, and technology with pristine customer service.
“Improving healthcare and delivering patient-centric solutions are key to both our customers and PCI as a whole,” Stokes asserts.
Having recently announced significant investments in advanced drug delivery and device combination capabilities, the company is truly delivering on its vision.
With device platform providers developing increasingly patient-centric technologies, PCI is proud to be leading the market with its services in the final assembly, packaging, and testing of advanced injectable products such as prefilled syringes, needle safety devices, and pens from clinical to commercial scale.
“With aiding compliance and patient safety at the core of our operations, the fact we are able to deliver end-to-end services from single sites with global reach has cemented our position in this market,” she shares.
As such, PCI is assisting in the global shift towards moving patient care from the hospital setting and into patients’ homes.
The company’s capabilities in this area are built from combining extensive experience, scientific knowledge, regulatory excellence, and industry-leading technologies to address the ever-increasing demand for patient-centric, convenient solutions that aid compliance and ultimately deliver improved health outcomes.

Meanwhile, PCI’s key therapeutic areas include oncology, the central nervous system (CNS), and autoimmune diseases, with advances being made across each.
In oncology, for example, a high proportion of the drug products at both the clinical development and commercial stages of the lifecycle are classified as highly potent.
In recent years, PCI has built and operationalised two high-potency development and manufacturing centres of excellence at its facility in the UK, which utilise the latest fully isolated containment technologies.
“These facilities enable the safe development and clinical and commercial supply of products with an occupational exposure limit as low as 0.01 micrograms per cubic metre.”
To further complement the manufacturing investments co-located at the same site, the company has also invested in a high-potency packaging facility to provide a seamless solution for its clients.
Recognising Australia as one of the most mature markets in the Asia Pacific region for clinical trials, with the Australian drug sector investing more than AUD$1 billion annually into R&D and outsourcing services, PCI has invested significantly in its clinical facilities in Melbourne to expand its capabilities and capacities for clinical labelling, packaging, storage, and distribution.
Managing clinical studies within Australia and across the Asia Pacific region with specialist capabilities in cold chain logistics and licenced to hold controlled drugs, PCI can support both local and global clients.
“The overarching vision for PCI and the driving force behind our strategy and the investments we make are fundamentally built on playing an integral part in improving health outcomes for patients,” Stokes declares.
“The overarching vision for PCI and the driving force behind our strategy and the investments we make are fundamentally built on playing an integral part in improving health outcomes for patients”
Michèle Stokes PhD GAICD, Vice President – Asia Pacific, PCI Pharma Services
SUSTAINABLE TO THE CORE
In line with its overall values and beliefs, PCI cultivates a global focus on robust environmental, social, and governance (ESG) standards.
“Through our ESG strategy, we are committed to fostering environmentally sustainable performance, honouring a diverse and inclusive culture, and positively impacting our employees, supply partners, customers, patients, and the communities in which we live and work,” Stokes divulges.
Whilst the company’s ESG principles are global, the power of its Community Impact Strategy can be felt closer to home.
By listening to the voices of local people and maintaining a continuous dialogue with them, PCI ensures that it remains connected to their evolving needs.
“We have established a formal giving-back strategy, focused on year-on-year increases in employee participation and measurable community impact for each of our global sites and corporate functions,” she elaborates.
As such, each site offers volunteer opportunities and encourages employees to create interest groups for a heightened feeling of connection.
To further support its strategy, PCI is formalising its ESG Day initiative this year, in which employees can log up to eight hours of company-sponsored volunteer activities per year.
“This initiative will encourage our staff to cultivate meaningful relationships with communities through philanthropic work and dedicate their time in ways that have a significant and measurable impact,” Stokes emphasises.
PCI seeks to deeply embed its sustainability initiatives across the business and has identified nine key focus areas to drive its global strategy, from waste management to energy efficiency, human rights, water conservation, and more.
“The reality is that the pharmaceutical industry today has a 55 percent higher carbon footprint than the automotive industry,” she acknowledges.
Therefore, the company deems it essential to work towards impactful change by striving to eventually use 100 percent renewable energy sources.
ONWARDS AND UPWARDS
To continue to meet the dynamic demands of clinical trials and commercial markets, PCI consistently evolves its offerings, whether for potent solid oral doses, sterile injectables, lyophilised drug products, or bespoke packaging of specialist advanced drug deliveries.
“Whether adding service offerings for new modalities or expanding our current capabilities and capacities, we will continue to provide a collaborative, creative, and tailored approach to ensure the delivery of life-changing therapies for patients,” Stokes confirms.
Successfully launching products on behalf of its global client base each year, PCI’s focus on key therapeutic areas and the investments it has made in this sphere will continue.
For Melbourne and Australia specifically, PCI’s goals include increasing its ultra-low temperature offerings to support the growing clinical trial market demand, introducing liquid nitrogen storage, adding additional storage, and doubling its labelling capacity, including cold chain labelling capacity.
With evolving capabilities in highly potent therapies, sterile fill-finish, lyophilisation, drug-device combination assembly, packaging and testing, cold chain infrastructure, storage, and distribution, the company is ideally placed to support clients in the years ahead.
“As well as this, we will continue to read the market, understand the trends and unmet needs, listen to our customers, and plan further investment strategies to work in partnership with our ever-growing client base,” Stokes concludes.